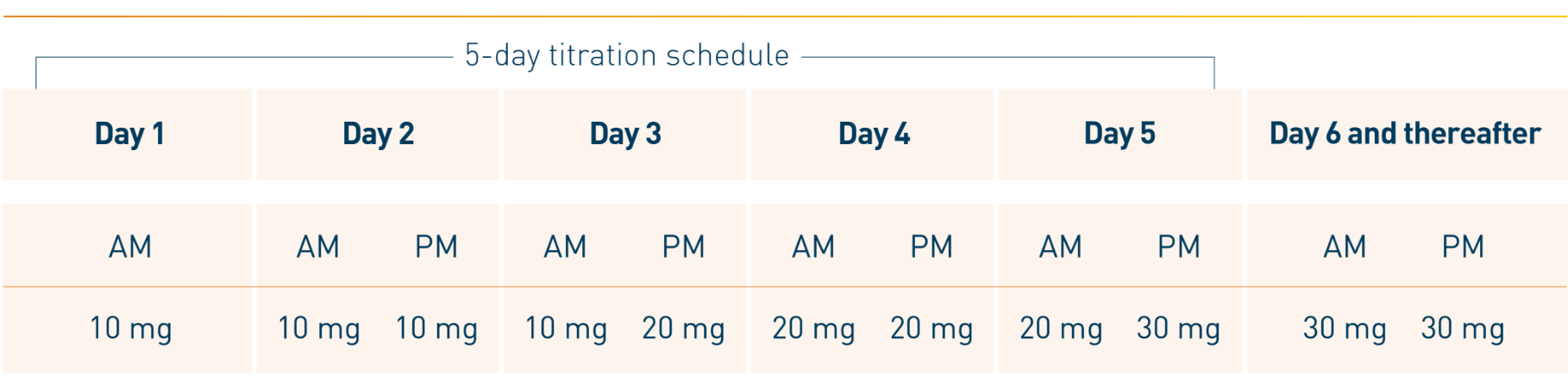
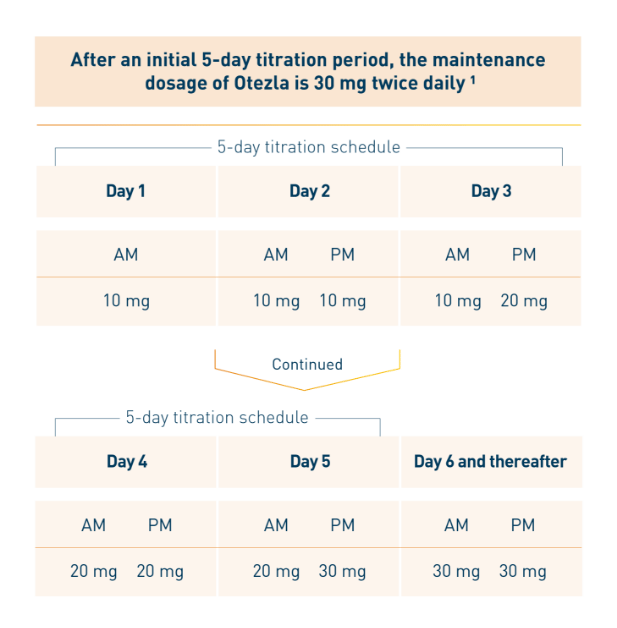
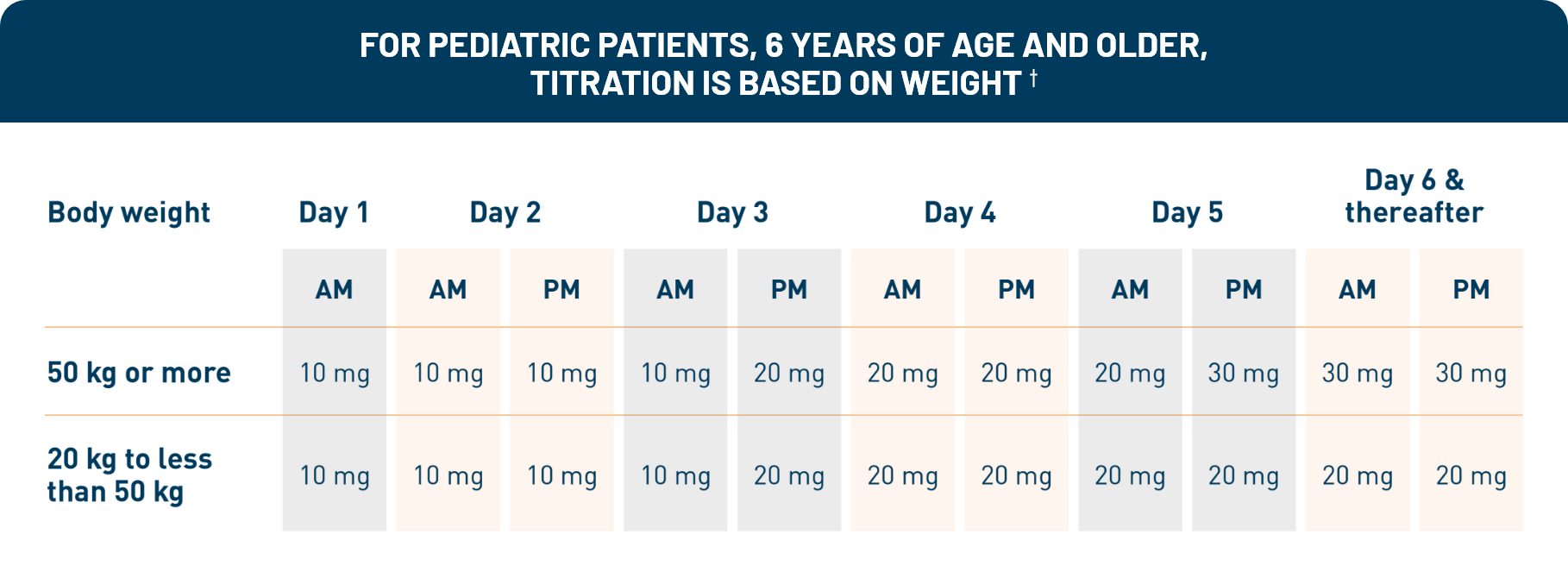
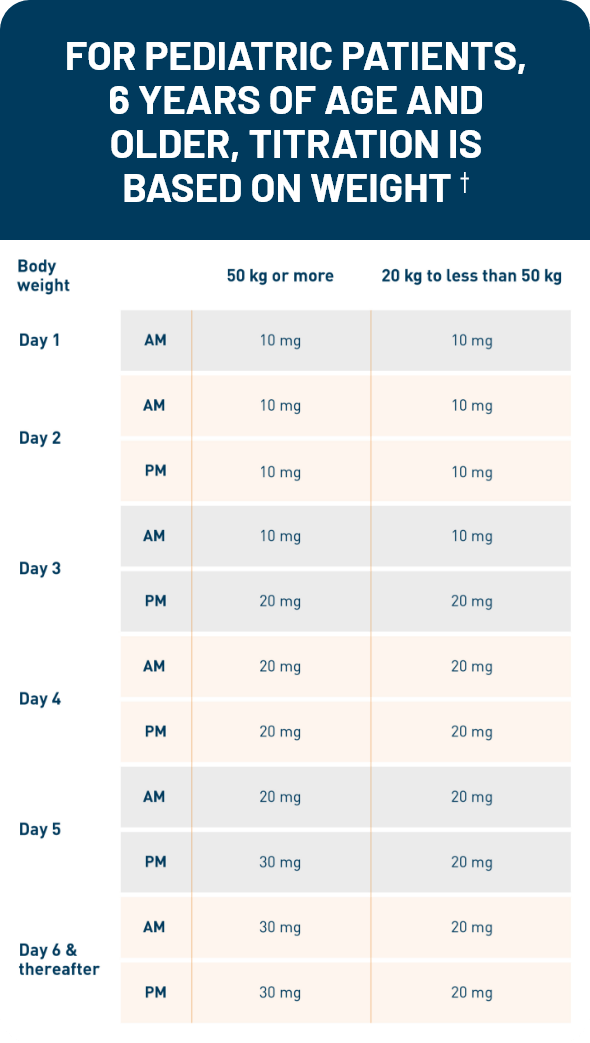
Otezla was evaluated in a robust clinical development program that began in 2010. Patients in the long-term open-label extension phase of the ESTEEM and PALACE clinical trials were followed for up to 5 years. 1,2
The safety and efficacy of Otezla in moderate to severe plaque psoriasis clinical trials were assessed in 1257 subjects in 2 multicenter, randomized, double-blind, placebo-controlled trials (Studies PSOR-1 and PSOR-2). In mild to moderate plaque psoriasis, the safety and efficacy of Otezla were evaluated in 595 subjects in a multicenter, randomized, placebo-controlled, double-blind study. In PsA clinical trials, the safety and efficacy of Otezla were evaluated in 1493 adult patients with active PsA in 3 multicenter, randomized, double-blind, placebo-controlled trials (Studies PsA-1, PsA-2, and PsA-3). 3
Plaque Psoriasis
Otezla was evaluated in a robust global clinical development program. 3-5
Pivotal Study: ADVANCE
ADVANCE was a multicenter, randomized, placebo-controlled, double-blind study of biologic-naïve adults with mild to moderate plaque psoriasis. 3
Pivotal Studies: ESTEEM 1 and ESTEEM 2
ESTEEM 1 and ESTEEM 2 were 2 large, pivotal, Phase 3, randomized, placebo-controlled studies evaluating apremilast in patients with a diagnosis of moderate to severe plaque psoriasis for at least 12 months prior to screening, and who were also candidates for phototherapy and/or systemic therapy. 1-3
Additional Studies: LIBERATE
LIBERATE was a global, Phase 3b, placebo-controlled, double-blind, double-dummy study that evaluated use of apremilast in biologic-naïve patients with moderate to severe plaque psoriasis for up to 104 weeks. 4
Additional Studies: STYLE
STYLE was a Phase 3, multicenter, randomized, double-blind, placebo-controlled study of 303 adult moderate to severe plaque psoriasis patients with moderate to severe plaque psoriasis of the scalp. 6
Additional Studies: DISCREET
DISCREET was a Phase 3, multicenter, randomized, placebo-controlled, double-blind study of 289 adult patients with moderate to severe plaque psoriasis and moderate to severe plaque psoriasis of the genital area. 3
Additional Studies: SPROUT
SPROUT was a Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel group
study to assess the efficacy and safety of Otezla in 245 pediatric patients whose ages ranged from
6 to 17 years old, with moderate to severe plaque psoriasis. 7
Psoriatic Arthritis
In multiple clinical trials, Otezla has been evaluated in more than 1400 patients with psoriatic arthritis. 8-10
Pivotal Studies: PALACE 1-3
PALACE 1, PALACE 2, and PALACE 3 were 3 pivotal, Phase 3, multicenter, randomized, double-blind, placebo-controlled studies in patients with active PsA, despite prior or current DMARD therapy. 3,8
Additional Studies: PALACE 4
PALACE 4 was a Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study of 527 csDMARD-naïve and biologic-naïve adult patients with active PsA. 10
Additional Studies: FOREMOST
FOREMOST is a global randomized, double-blind, placebo-controlled, parallel-group, Phase 4 study that
uniquely focused on early oligoarticular PsA. Patients with oligoarticular PsA (>1 but ≤4 tender and swollen
joints involved) and early disease (signs and symptoms of PsA ≤5 years’ duration) were randomized 2:1 to
receive either Otezla 30 mg BID (n=203) or placebo (n=105) for 24 weeks, stratified based on concomitant
medication use, with an early escape at week 16.11,12
Additional Studies: ACTIVE
ACTIVE was a global, Phase 3b, multicenter, randomized, double-blind, placebo-controlled, parallel-group
study evaluating apremilast monotherapy in adult patients with active PsA who were biologic naïve for up
to 104 weeks. 9
Oral Ulcers in Behçet’s Disease
Otezla was evaluated in adult patients with Behçet’s Disease (BD) with active oral ulcers. 3
Pivotal Study: RELIEF
Otezla was evaluated in a Phase 3, multicenter, randomized, double-blind, placebo-controlled trial of 207 adult patients with BD and active oral ulcers. 3,13
BID, twice daily; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DMARD, disease-modifying antirheumatic drug;
PsA, psoriatic arthritis.
References: 1. Papp K, Reich K, Leonardi CL, et al. J Am Acad Dermatol. 2015;73(1):37-49.
2. Paul C, Cather J, Gooderham M, et al. Br J Dermatol. 2015;173(6):1387-1399.
3. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc.
4. Reich K, Gooderham M, Green L, et al. J Eur Acad Dermatol
Venereol. 2017;31(3):507-517.
5. Stein Gold L, Papp K, Pariser D, et al. J Am Acad Dermatol. 2022;86(1):77-85.
6. Van Voorhees AS, Stein Gold L,
Lebwohl M, et al. J Am Acad Dermatol. 2020;83(1):96-103.
7. Fiorillo L, Becker E, de Lucas R, et al. J Am Acad Dermatol. 2024;90(6):1232-1239.
8. Kavanaugh A, Gladman DD, Edwards CJ, et al. Arthritis Res Ther. 2019;21(1):118.
9. Nash P, Ohson K, Walsh J, et al; ACTIVE Investigators. Ann Rheum Dis. 2018;77(5):690-698.
10.
Wells AF, Edwards CJ, Kivitz AJ, et al.
Rheumatology (Oxford). 2018;57(7):1253-1263.
11. Mease P, Gladman D, Coates LC, et al. Presented at: ACR/ARHP Annual Meeting; November 10-15, 2023; San
Diego, CA.
12. Gossec L, Gladman D, Coates L, et al. Presented at: 32nd Annual Meeting of the European Academy of Dermatology and
Venereology (EADV); October 11-14, 2023; Berlin, Germany.
13. Hatemi G, Mahr A, Ishigatsubo Y, et al. N Engl J Med. 2019;381(20):1918-1928.