First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
OTEZLA:
4 INDICATIONSOtezla® (apremilast)/Otezla XR™ (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy.Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
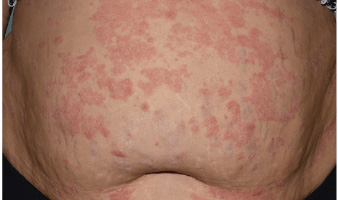
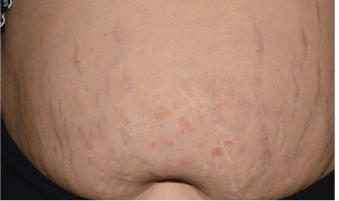
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
*PASI-49.7 response: A 49.7% reduction in a patient’s PASI score. 1
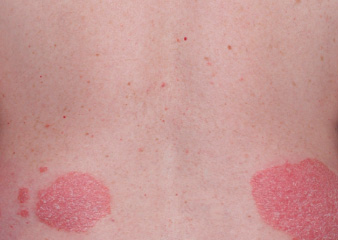
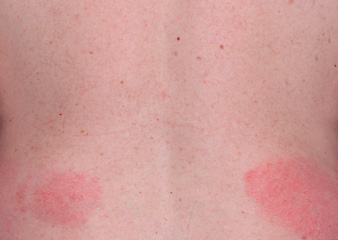
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
†PASI-52 response: A 52% reduction in a patient’s PASI score. 1
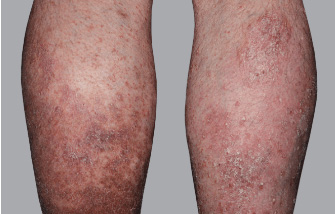
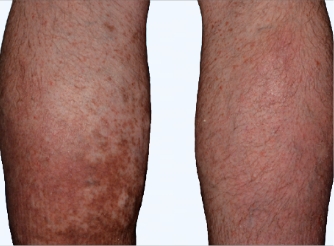
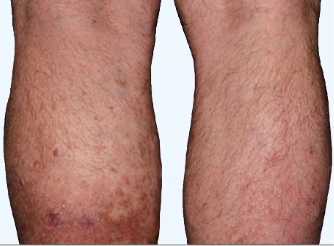
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
‡PASI-62.5 response: A 62.5% reduction in a patient’s PASI score. 1
§PASI-72 response: A 72% reduction in a patient’s PASI score. 1
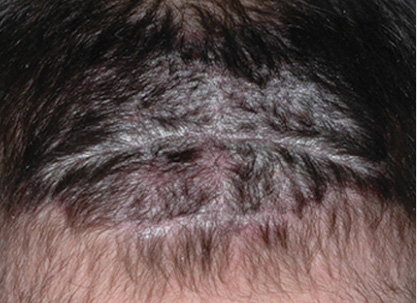
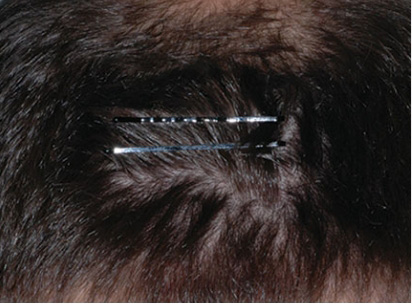
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
**PASI-63 response: A 63% reduction in a patient’s PASI score. 1
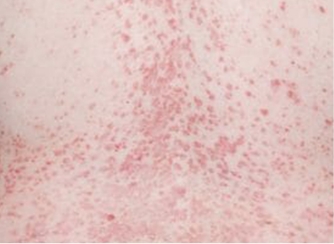
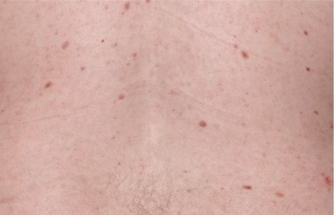
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
††PASI-69 response: A 69% reduction in a patient’s PASI score. 1
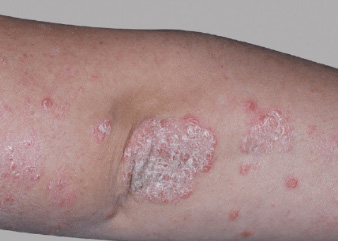
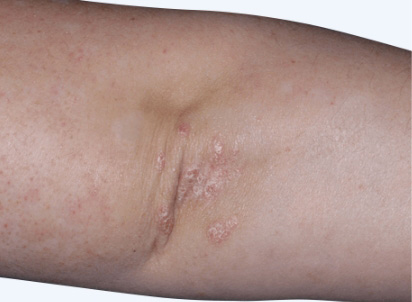
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
‡‡PASI-70 response: A 70% reduction in a patient’s PASI score. 1
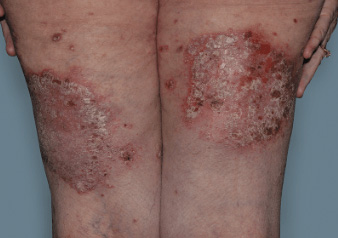
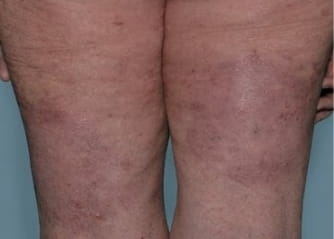
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
§§PASI-75 response: A 75% reduction in a patient’s PASI score. 1
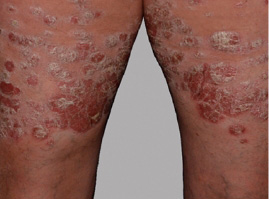
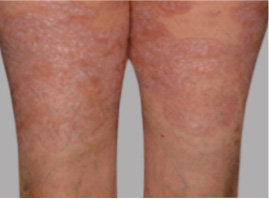
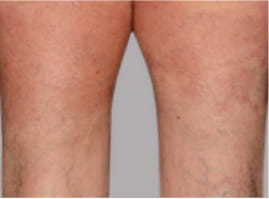
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
***PASI-76.5 response: A 76.5% reduction in a patient’s PASI score. 1
†††PASI-90 response: A 90% reduction in a patient’s PASI score. 1
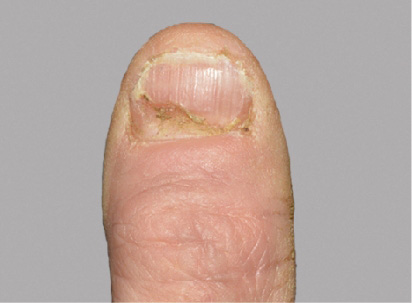
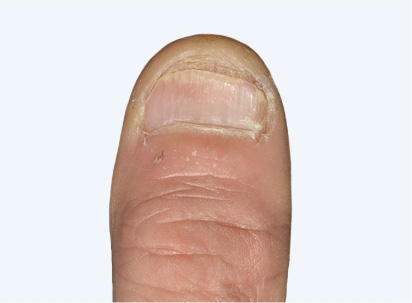
Actual Otezla® (apremilast) patient. 1 Images are not reflective of NAPSI score. Individual results may vary.
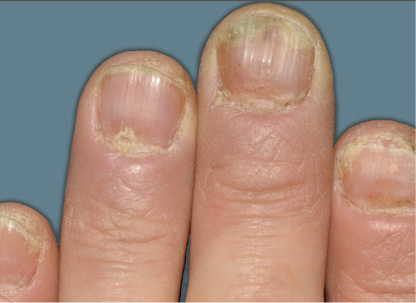
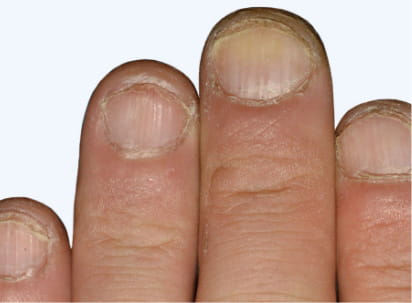
Actual Otezla® (apremilast) patient. 1 Images are not reflective of NAPSI score. Individual results may vary.
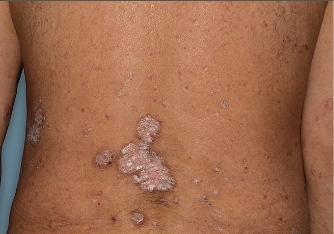
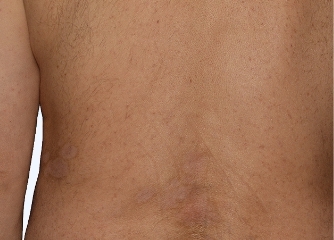
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
‡‡‡PASI-80 response: An 80% reduction in a patient’s PASI score. 1
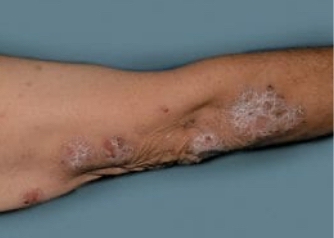
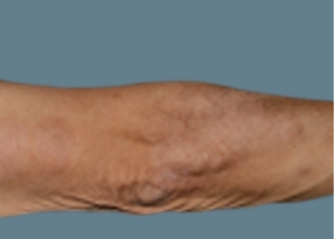
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
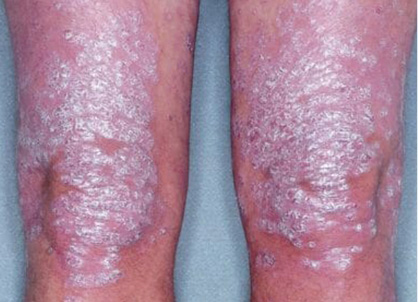
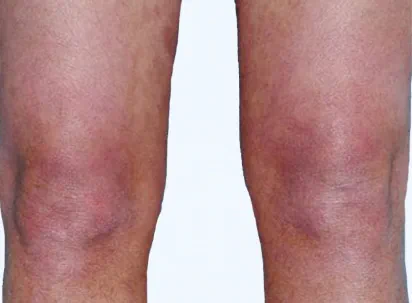
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
§§§PASI-85 response: An 85% reduction in a patient’s PASI score. 1
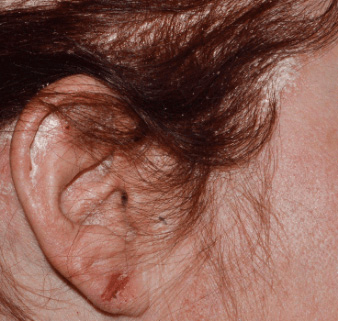
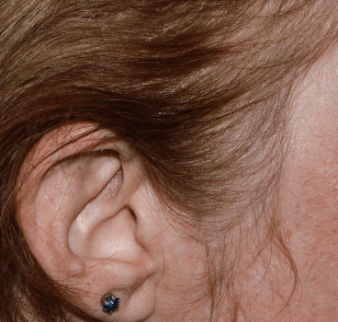
Actual clinical trial patient from STYLE. 1
Individual results may
vary.
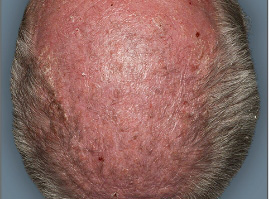
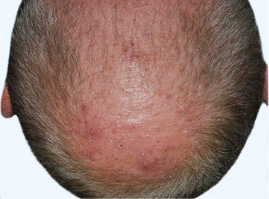
Actual clinical trial patient from STYLE. 1
Individual results may
vary.
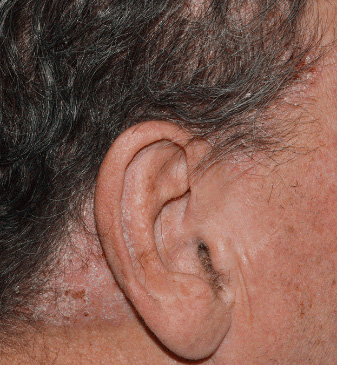
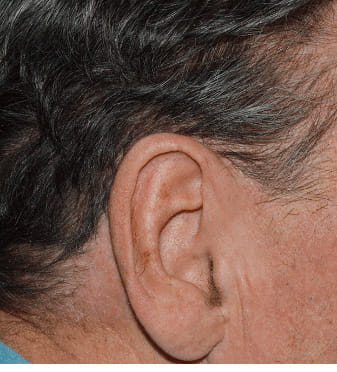
Actual clinical trial patient from STYLE. 1 Individual results may vary.
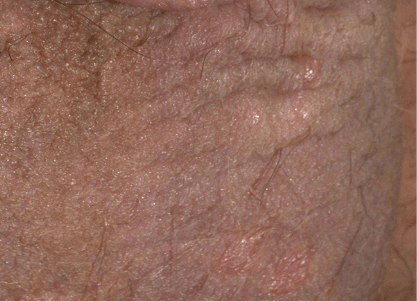
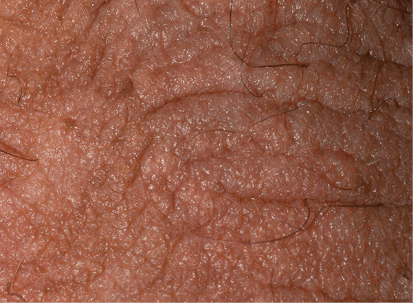
Actual clinical trial patient from DISCREET. 1 Patient received 16 weeks of Otezla treatment.
Patients
had sPGA-G improvement from moderate to mild. Individual results may vary.
NAPSI, Nail Psoriasis Severity Index; PASI, Psoriasis Area and Severity Index; ScPGA, Scalp Physician Global Assessment; sPGA-G, static Physician Global Assessment of Genitalia.
Contraindications
Otezla/OTEZLA XR is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity: Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla/OTEZLA XR and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Please click here for the full Prescribing Information.
Reference: 1. Data on file, Amgen; 2016.