First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
AN ESTABLISHED SAFETY PROFILE IN BIOLOGIC-NAÏVE PATIENTS 1
ACTIVE: Most common adverse reactions
(Reported in ≥5% of patients) 1
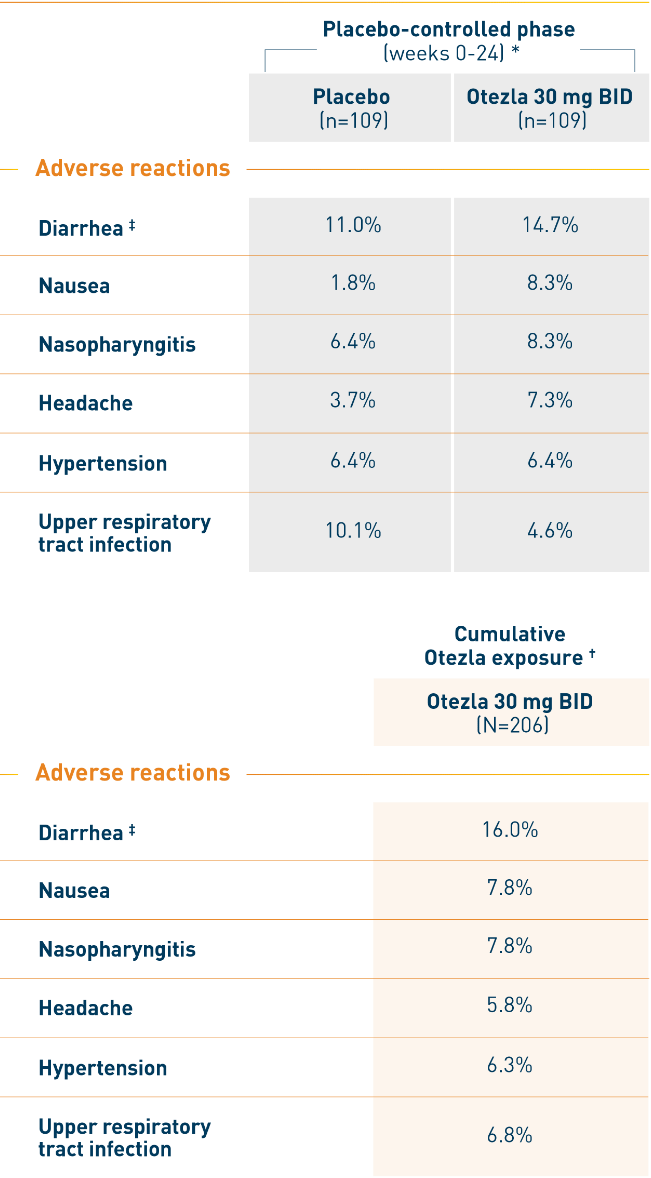
*Includes data through week 16 for placebo patients who escaped, and data through week 24 for all other patients. †Includes all available Otezla exposure data (including data beyond 52 weeks): patients with multiple reports are only counted once. ‡Protocol-defined characterization of diarrhea of 2 or more watery or liquid stools/day: placebo (8.3%) and Otezla 30 mg BID (11%).




