First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
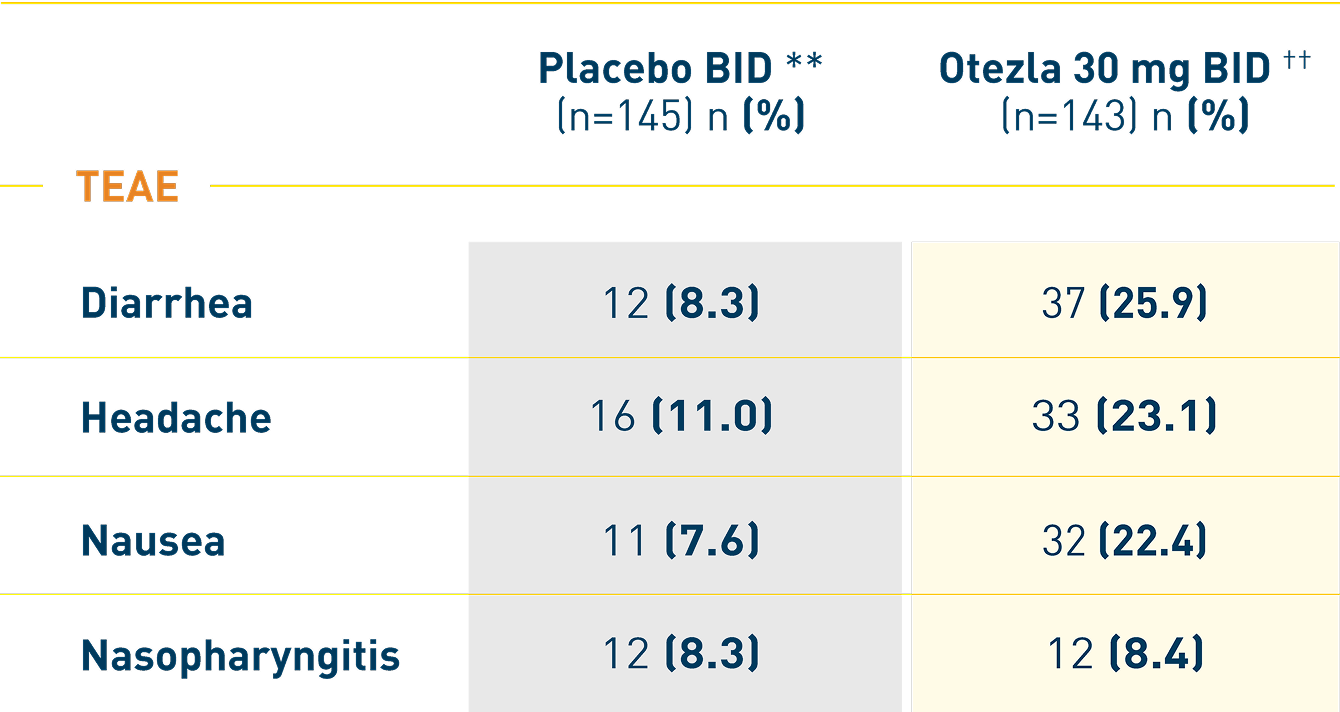
ESTEEM: Adverse reactions reported in ≥1% of subjects on Otezla
and with greater frequency than in subjects on placebo through week 16 2,*

*The safety of Otezla was assessed in 1426 subjects in 3 randomized, double-blind, placebo-controlled trials (including the ESTEEM studies). †Two subjects treated with Otezla experienced a serious adverse reaction of abdominal pain.
- Discontinuation of treatment due to any adverse reaction was 6.1% for patients taking Otezla and 4.1% for placebo 2
- The most common adverse reactions leading to discontinuation for patients taking Otezla were nausea (1.6%), diarrhea (1.0%), headache (0.8%) 2