First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
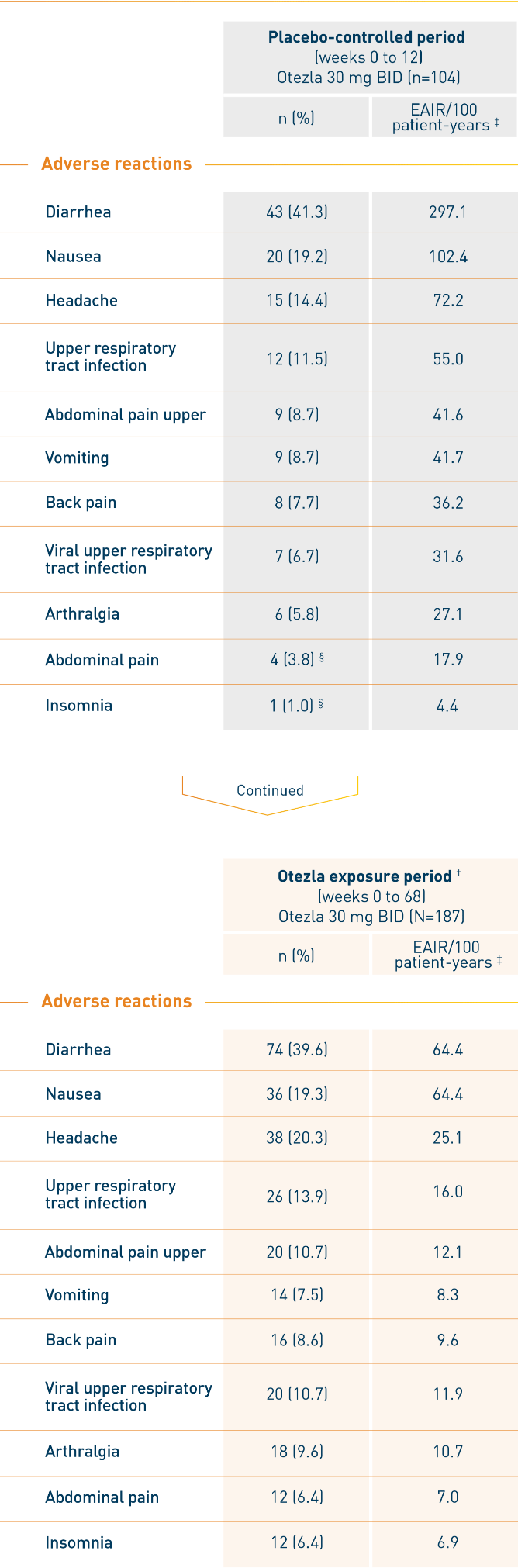
AN ESTABLISHED SAFETY PROFILE THROUGH WEEK 12 1
RELIEF: Adverse reactions reported in ≥5% of patients on Otezla 30 mg BID and
with at least 1% greater frequency than patients on placebo up to week 12 1
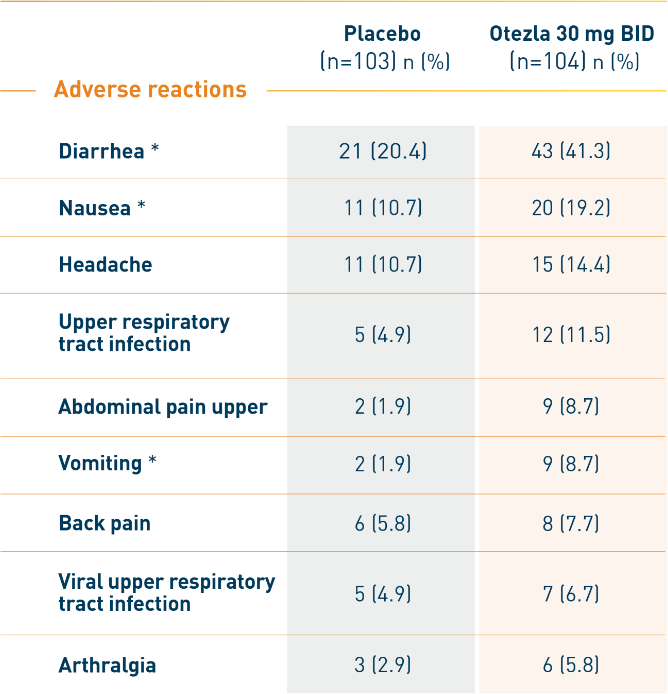
*There were no serious adverse reactions of diarrhea, nausea, or vomiting.
- The most commonly reported adverse reactions (≥10%) for Otezla include diarrhea, nausea, headache, and upper respiratory tract infection 1
- Discontinuation of treatment due to adverse reactions during the placebo-controlled period of the study was 2.9% for patients taking Otezla and 4.9% for placebo 1