First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
RESULTS SEEN IN OTEZLA PATIENTS
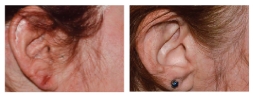
BASELINE
WEEK 16
ScPGA: 0
3-POINT
IMPROVEMENT IN
ScPGA SCORE
3-POINT
IMPROVEMENT IN
ScPGA SCORE
Actual clinical trial patient from STYLE. 4
Individual results may vary.
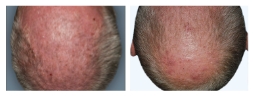
BASELINE
WEEK 16
ScPGA: 2
1-POINT IMPROVEMENT IN ScPGA SCORE
1-POINT IMPROVEMENT IN ScPGA SCORE
Actual clinical trial patient from STYLE. 4
Individual results may vary.
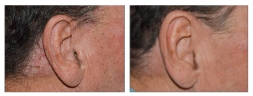
BASELINE
WEEK 16
ScPGA: 3
1-POINT IMPROVEMENT IN ScPGA SCORE
1-POINT IMPROVEMENT IN ScPGA SCORE
Actual clinical trial patient from STYLE. 4
Individual results may vary.