First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
THE FULL PRESCRIBING INFORMATION FOR OTEZLA HAS NO LAB MONITORING 1
RELIEF: Selected marked abnormalities in laboratory parameters
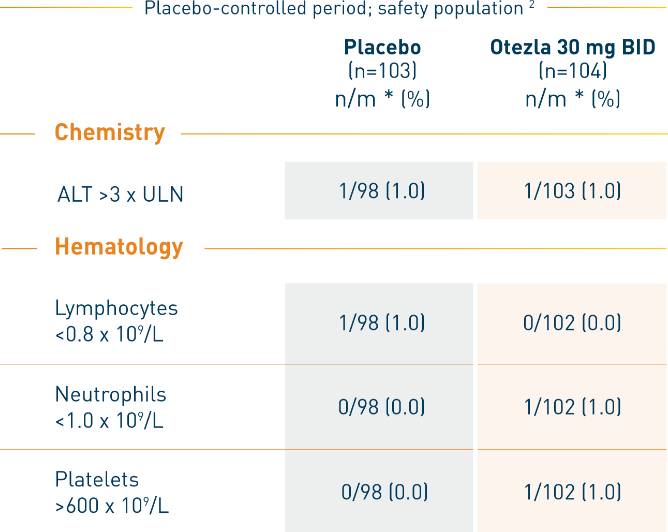
*n/m, number of patients with at least 1 occurrence of the abnormality/number of patients with a baseline value and at least 1 post-baseline value for criteria requiring baseline or number of patients with at least 1 post-baseline value for criteria not requiring baseline. Data collected later than 28 days after the last dose of Otezla were excluded. Patient incidence was 100 times n divided by m. Laboratory test results with no abnormalities were excluded from the table.




