First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
OTEZLA:
4 INDICATIONSOtezla® (apremilast)/Otezla XR™ (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy.Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
Study Design: Evaluated in adult patients with mild to moderate plaque psoriasis (N=595); age ≥18 years, sPGA score 2-3, BSA involvement 2%-15%, PASI score 2-15.1,2
Primary Endpoint: 22% of patients taking Otezla 30 mg BID (n=297) achieved an sPGA score of 0 (clear) or 1 (almost clear) and a ≥2-point reduction from baseline vs 4% with placebo (n=298) at week 16 (P<0.0001; ITT population).1,2

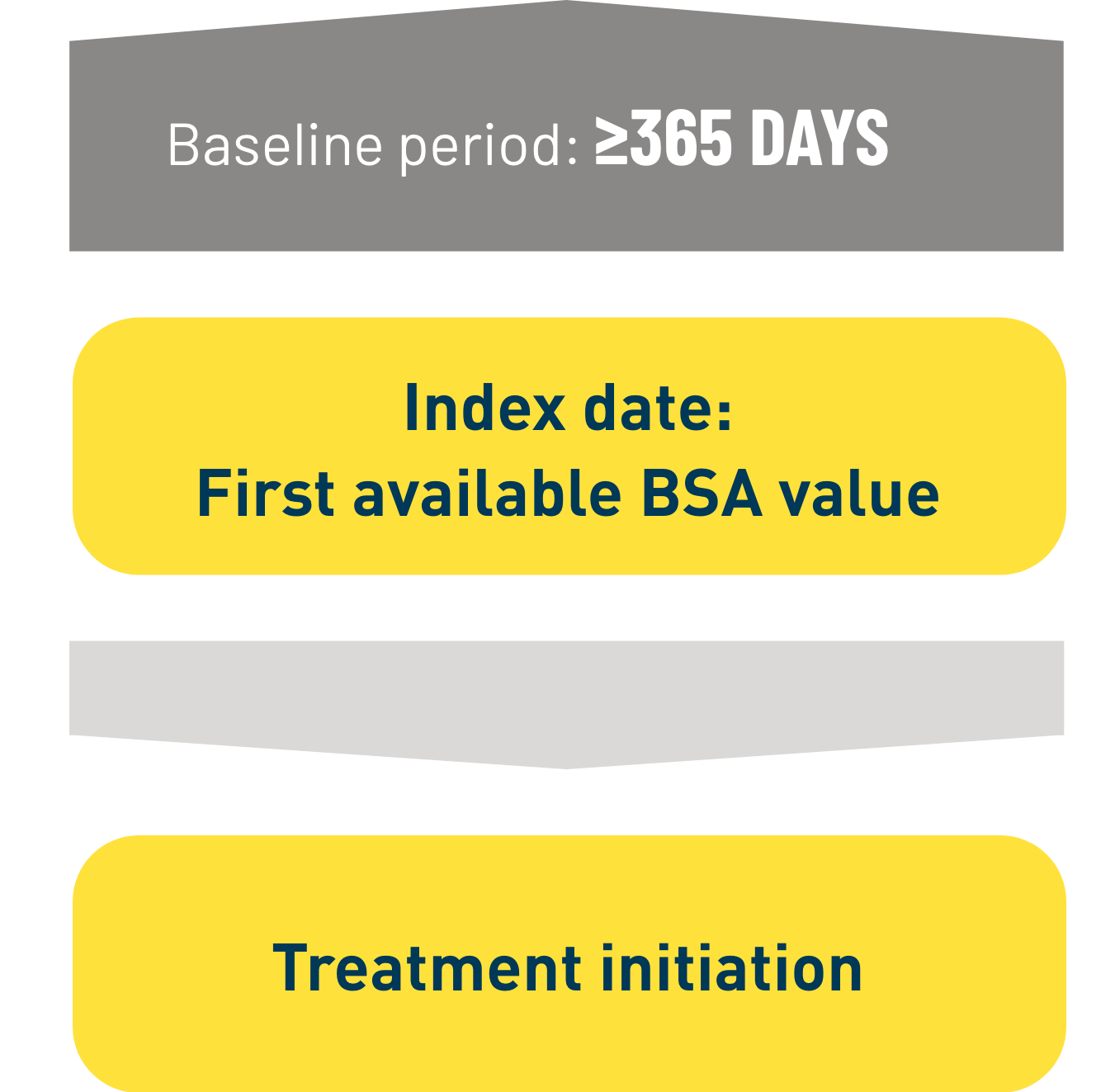
Design: Retrospective observational analysis based on electronic medical record and claims data in the OM1® database. 3
Patients: Adult patients with mild to moderate plaque psoriasis in the US who: 3
Select baseline disease characteristics in patients who started Otezla: Mean BSA 5.5% 3
Patients in the Otezla arm were permitted to continue topical therapy during the study. 4 50.5% of all
Otezla patients were using concomitant topical therapy. 4
n=2073
Started Otezla ≤6 months after index date
Median time to Otezla start:

n=1516
Started Otezla >6 months after index date
Median time to Otezla start:

n=9777
Initiated a second topical prescription between 2014 and 2023 at or after the index date
Median time to second type of topical start:

Limitations of this study include: 4


54% of early initiators had cycled through 3 or more topical therapies prior to starting Otezla 4,*
75% of late initiators had cycled through 3 or more topical therapies prior to starting Otezla 4,*
*Number of distinct topicals prior to Otezla initiation.
Please review the placebo-controlled data: Real-world evidence is not derived from a controlled clinical study and no conclusions of statistic or clinical significance can be drawn.
When your patients struggle on topical therapy,
BID, twice daily; BSA, body surface area; ITT, intent to treat; PASI, Psoriasis Area and Severity Index; PsO, plaque psoriasis; sPGA, static Physician Global Assessment.
Contraindications
Otezla/OTEZLA XR is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity: Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla/OTEZLA XR and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Please click here for the full Prescribing Information.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc.
2. Stein Gold L, Papp K, Pariser D, et al. J Am Acad Dermatol. 2022;86(1):77-85.
3. Strober B, Stein Gold L, Gisondi P, et al. Presented at: 7th World Psoriasis & Psoriatic Arthritis Conference; June 27-29, 2024; Stockholm, Sweden.
4. Data on file, Amgen; 2024.
5. Strober B, Stein Gold L, Gisondi P, et al. Presented at: Maui Derm Hawaii; January 22-26, 2024; Maui, HI.