First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
4 INDICATIONS Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy. Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=59% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
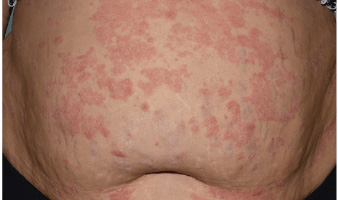
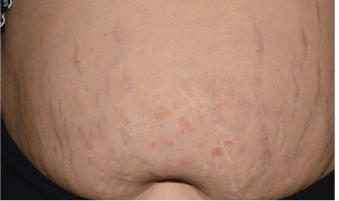
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
*PASI-49.7 response: A 49.7% reduction in a patient’s PASI score. 1
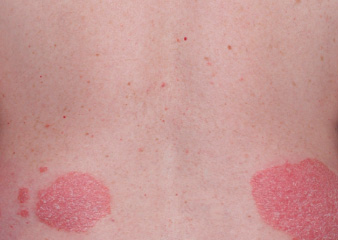
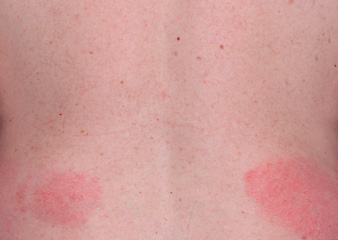
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
†PASI-52 response: A 52% reduction in a patient’s PASI score. 1
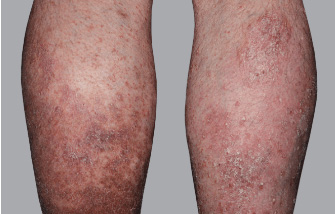
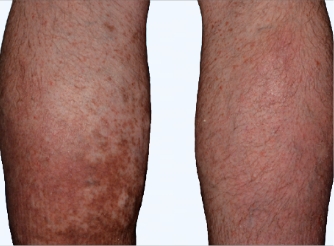
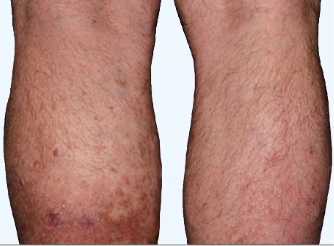
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
‡PASI-62.5 response: A 62.5% reduction in a patient’s PASI score. 1
§PASI-72 response: A 72% reduction in a patient’s PASI score. 1
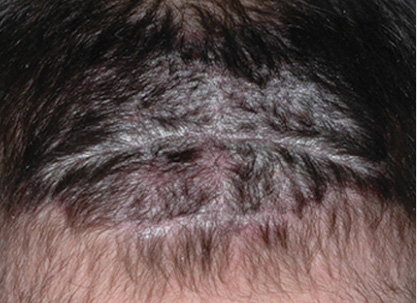
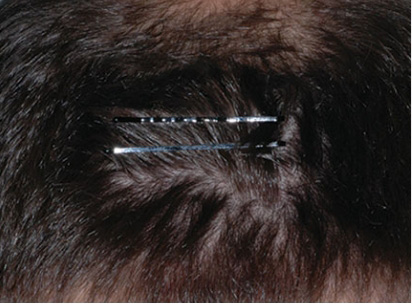
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
**PASI-63 response: A 63% reduction in a patient’s PASI score. 1
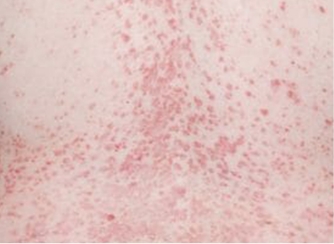
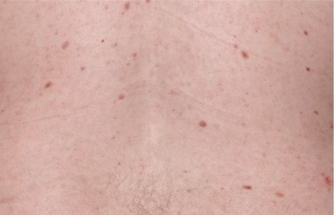
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
††PASI-69 response: A 69% reduction in a patient’s PASI score. 1
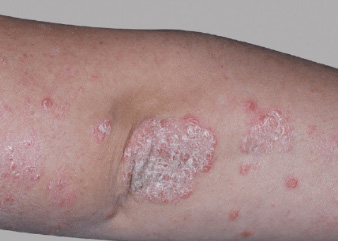
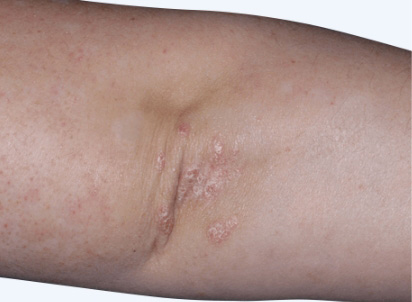
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
‡‡PASI-70 response: A 70% reduction in a patient’s PASI score. 1
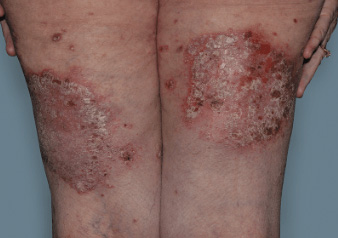
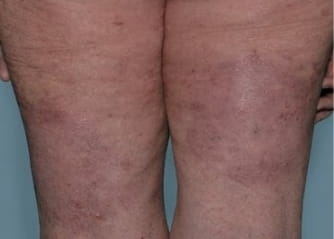
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
§§PASI-75 response: A 75% reduction in a patient’s PASI score. 1
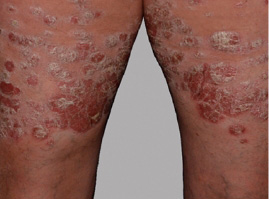
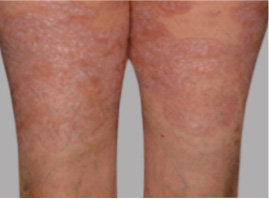
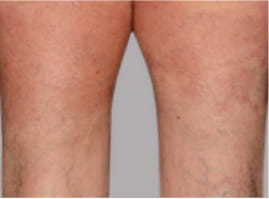
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
***PASI-76.5 response: A 76.5% reduction in a patient’s PASI score. 1
†††PASI-90 response: A 90% reduction in a patient’s PASI score. 1
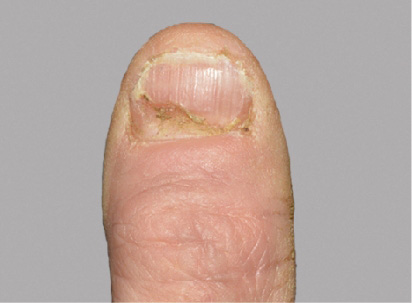
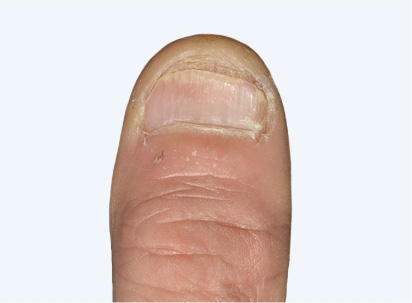
Actual Otezla® (apremilast) patient. 1 Images are not reflective of NAPSI score. Individual results may vary.
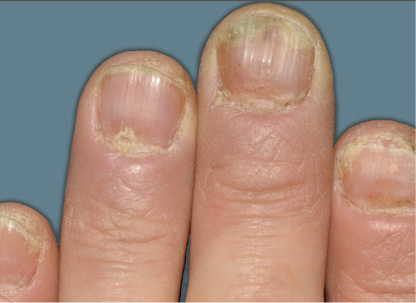
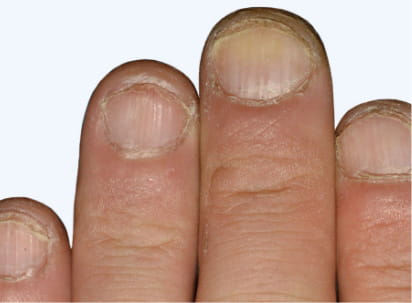
Actual Otezla® (apremilast) patient. 1 Images are not reflective of NAPSI score. Individual results may vary.
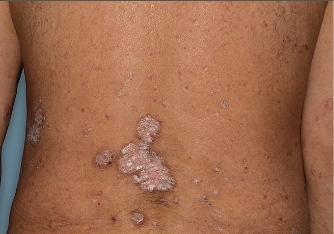
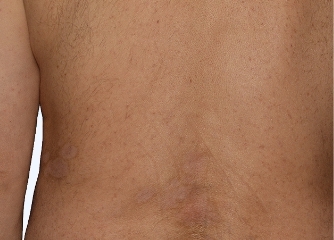
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
‡‡‡PASI-80 response: An 80% reduction in a patient’s PASI score. 1
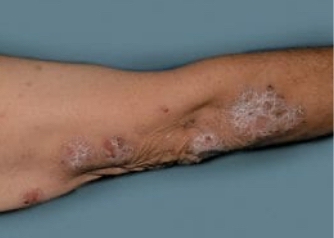
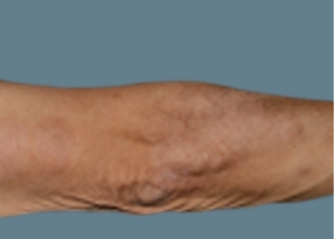
Actual Otezla® (apremilast) patient. 1 Individual results may vary.
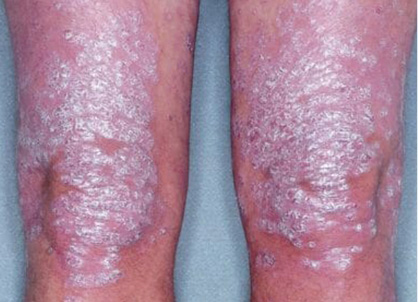
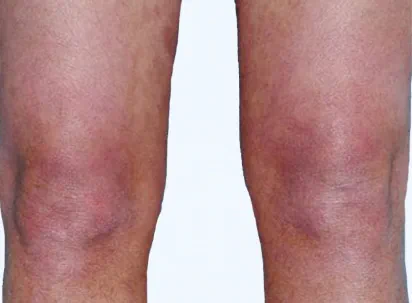
Actual clinical trial patient from ESTEEM. 1 Individual results may vary.
§§§PASI-85 response: An 85% reduction in a patient’s PASI score. 1
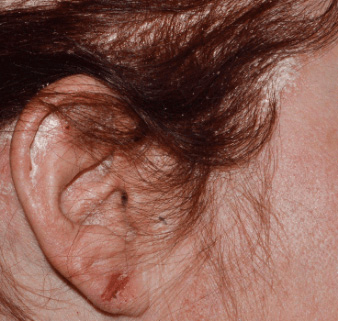
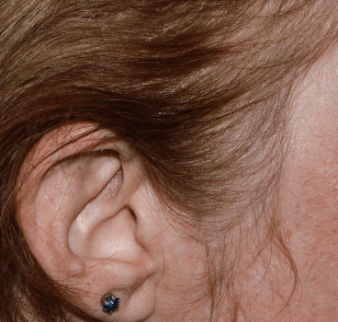
Actual clinical trial patient from STYLE. 1
Individual results may
vary.
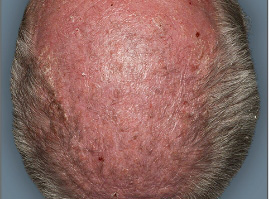
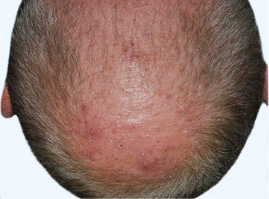
Actual clinical trial patient from STYLE. 1
Individual results may
vary.
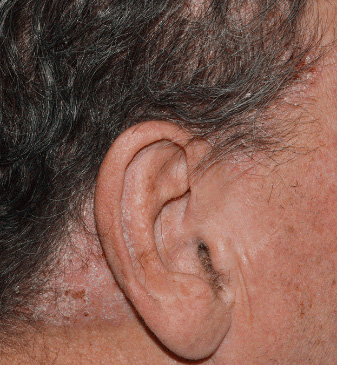
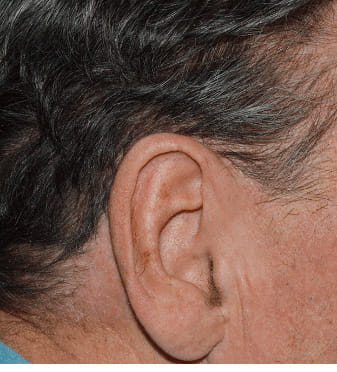
Actual clinical trial patient from STYLE. 1 Individual results may vary.
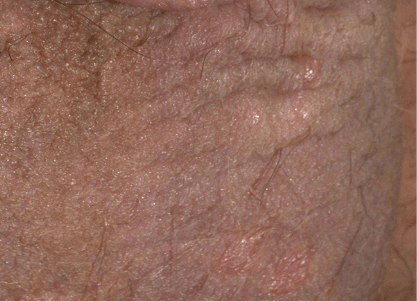
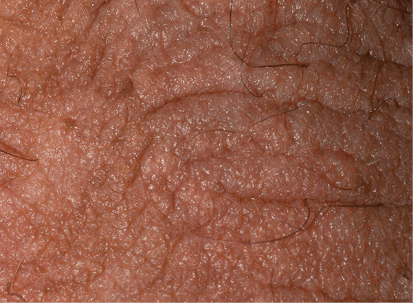
Actual clinical trial patient from DISCREET. 1 Patient received 16 weeks of Otezla treatment.
Patients
had sPGA-G improvement from moderate to mild. Individual results may vary.
NAPSI, Nail Psoriasis Severity Index; PASI, Psoriasis Area and Severity Index; ScPGA, Scalp Physician Global Assessment; sPGA-G, static Physician Global Assessment of Genitalia.
Contraindications
Otezla® (apremilast) is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Otezla is indicated for the treatment of:
Please click here for the full Prescribing Information.
Reference: 1. Data on file, Amgen; 2016.