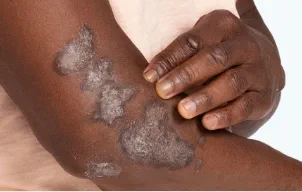
First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA
OTEZLA HAS BEEN STUDIED ACROSS ALL SEVERITIES OF
PLAQUE PSORIASIS AND IN ACTIVE PSORIATIC ARTHRITIS
TREAT MULTIPLE MANIFESTATIONS OF
PLAQUE PSORIASIS WITH OTEZLA 1-3,5


OTEZLA IS ALSO APPROVED
TO TREAT ACTIVE
PSORIATIC ARTHRITIS 1
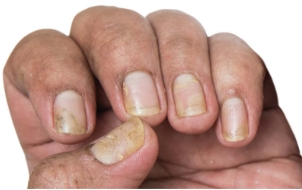
*Efficacy of Otezla in nail psoriasis was studied in the ADVANCE clinical trial for patients with mild to moderate plaque psoriasis. Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
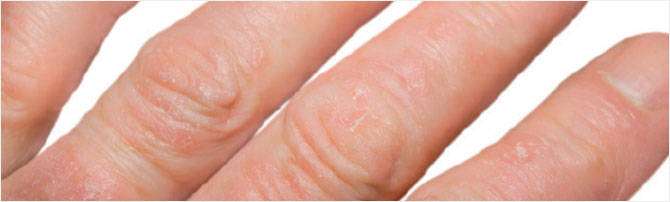
STUDY RESULTS: MILD TO MODERATE PLAQUE PSORIASIS
- In ADVANCE, 22% of patients taking Otezla (n=297) achieved an sPGA score of 0 (clear) or 1 (almost clear) and a ≥2-point reduction from baseline vs 4% with placebo (n=298) at week 16 (P<0.0001; primary endpoint; ITT population) 1,2
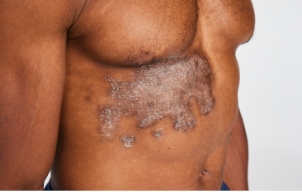
STUDY RESULTS: MODERATE TO SEVERE PLAQUE PSORIASIS
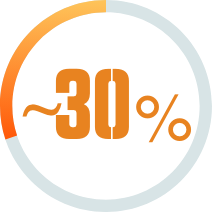
- In ESTEEM 1, 33% of patients taking Otezla (n=562) achieved PASI-75 response vs 5% with placebo (n=282) at week 16 (P<0.0001; primary endpoint; LOCF; FAS) 3,6
- In ESTEEM 2, 29% of patients taking Otezla (n=274) achieved PASI-75 response vs 6% with placebo (n=137) at week 16 (P<0.001; primary endpoint) 4
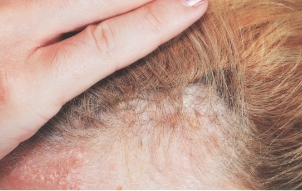
- In STYLE, 43% of patients taking Otezla (n=201) achieved a significant improvement in ScPGA response vs 14% with placebo (n=102) at week 16 (P<0.0001; primary endpoint; ITT population; MI analysis) 6
- In DISCREET, 40% of patients taking Otezla (n=143) achieved significant improvement in modified sPGA-G response, vs 20% with placebo (n=146) at week 16 (P=0.0003; primary endpoint) 1,5
- In SPROUT, 33% (n=163) of patients taking Otezla achieved an sPGA score of 0 (clear) or 1 (almost clear) and a ≥2-point reduction from baseline vs 10.8% placebo (n=82) at week 16 (P<0.0001; primary endpoint) 1,5