First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
3 INDICATIONS Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy. Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=58% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
*Patients were given Otezla 30 mg BID. 65% of patients received concomitant therapy with at least 1 DMARD, including 55% methotrexate.
At week 16, the respective percentages of patients with ACR50
and ACR70 responses were as follows 2,3,†:
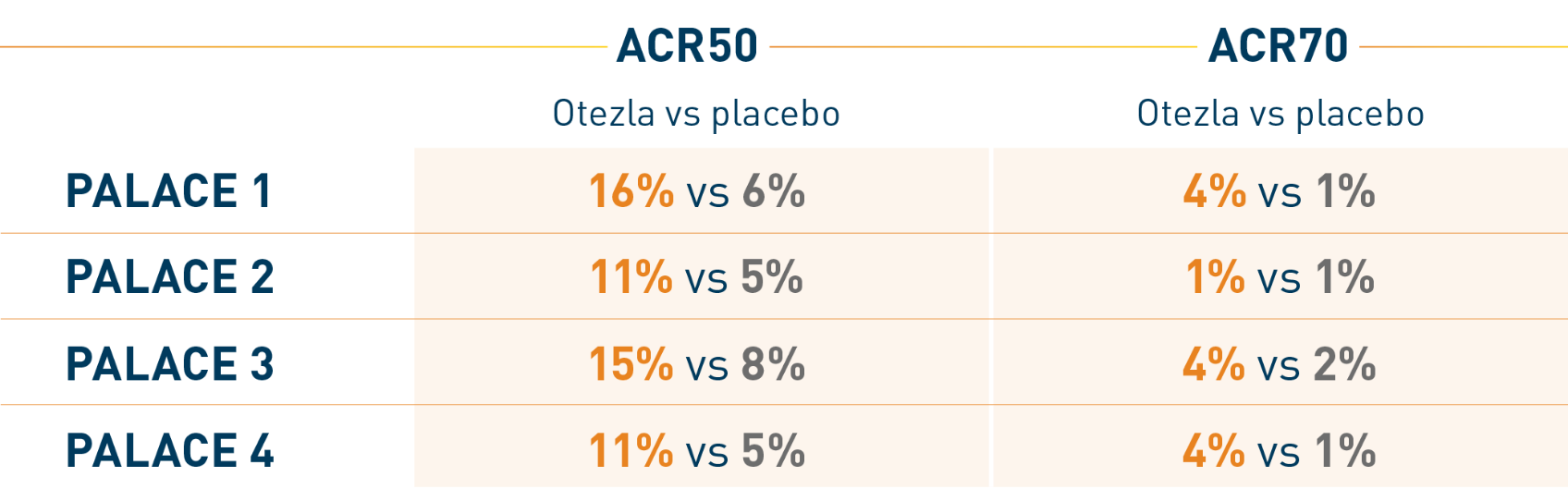
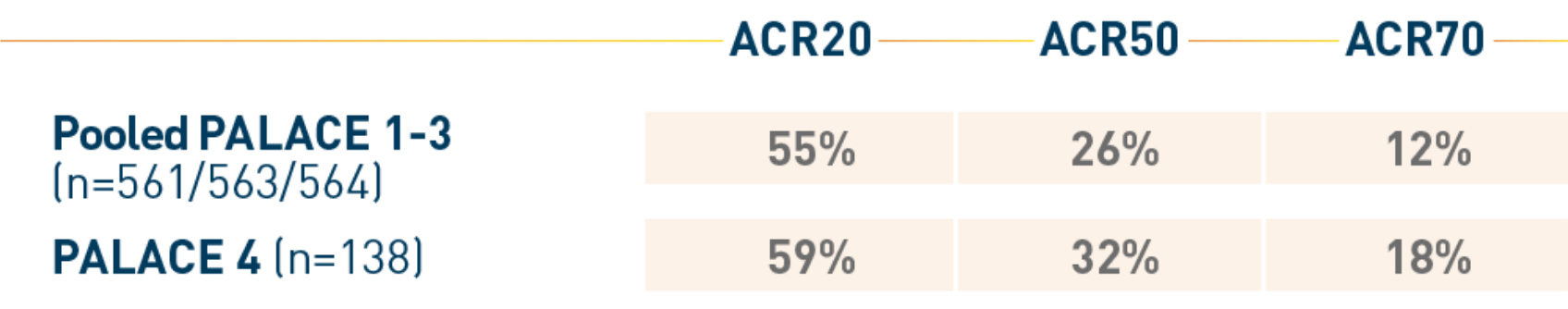
At week 52, the respective percentages of patients taking Otezla
30 mg BID with ACR50 and ACR70 responses were as follows 2,†:
†Did not reach statistical significance.
This analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
— MEASE ET AL, 2022
‡DAPSA is comprised of 5 components: tender joint count; swollen joint count; Patient Assessment of Pain (PAP); Patient Global Assessment of Disease Activity
(PtGA); C-reactive protein.
§Clinical remission does not mean drug-free remission or complete absence of disease.
VIDEO TRANSCRIPT

Dr. Leventhal: Hi, I’m Dr. Lawrence J. Leventhal, Rheumatologist at Holy Redeemer Hospital and Medical Center, and today I would like to discuss a treat-to-target approach for your patients with psoriatic arthritis, using the Clinical Disease Activity Index for Psoriatic Arthritis, or cDAPSA. Current guidelines, such as the American College of Rheumatology and National Psoriasis Foundation guidelines, recommend a treat-to-target approach when managing patients with psoriatic arthritis. 1 A treat-to-target approach entails regularly assessing an outcome and adjusting therapies, as needed, to reach a target disease activity level. 2 Multiple tools can be used to assess outcomes, such as minimal disease activity, or MDA, and the Disease Activity Index for Psoriatic Arthritis, or DAPSA. 1 Today, I would like to elaborate on cDAPSA, which is a tool that measures disease activity based on 4 components that primarily focus on joint manifestations. 3 The cDAPSA total score is a combination of tender joint count, swollen joint count, patient assessment of pain, and patient global assessment of disease activity. 3 The score can range from 0 to 154 and includes 4 categories. A score of 0 to 4 represents remission, 5-13 represents low disease activity, 14-27 represents moderate disease activity, and 28-154 represents high disease activity. 3,4 I hope this short presentation helped you better understand a treat-to-target approach using the cDAPSA tool in managing patients with psoriatic arthritis. Thank you!
References: 1. Singh, et al. Arthritis Rheumatol. 2019;71:5-32. 2. Ogdie, et al. Rheumatology (Oxford). 2020;59:i37-i46. 3. Schoels, et al. Ann Rheum Dis. 2016;75:811-818. 4. Mease, et al. Arthritis Care Res (Hoboken). 2020;72:814-821.
Post-hoc analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
**cDAPSA was calculated as a composite score based on SJC, TJC, PAP, and PtGA with possible scores ranging from 0-154. ††The post-hoc analyses included patients
from the PALACE 1, 2, 3, and 4 clinical trials receiving Otezla 30 mg BID who had undergone randomization at baseline and had cDAPSA components available to
calculate responses (PALACE 1-3, n=494; PALACE 4, n=175). ‡‡REM or LDA. §§Clinical remission does not mean drug-free remission or complete absence of disease.
***Includes patients who had undergone randomization and who had available cDAPSA components at baseline (n=494).
VIDEO TRANSCRIPT

Dr. Sulich: Hello! I’m Dr. Andrew Sulich. Let’s review the PALACE 1-3 results and cDAPSA post-hoc analysis in adult patients with active psoriatic arthritis, which identified patients who are most likely to achieve treatment targets. 1-4 Otezla® , apremilast, is an oral, nonbiologic PDE4 inhibitor indicated for the treatment of adult patients with active psoriatic arthritis. 5 It is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation. 1493 total patients were involved in PALACE 1-3 trials. During the placebo-controlled phase, patients were randomized to receive Otezla 20 mg or 30 mg twice daily or placebo. The primary endpoint was the percentage of patients achieving an American College of Rheumatology criteria for an at least 20% improvement, or ACR20 response, at week 16. 5,6 Significantly more patients receiving Otezla achieved ACR20 response compared with the placebo at week 16 in PALACE 1. Similar results were seen in PALACE 2 and 3. 5 At week 16, the ACR50 and ACR70 responses did not reach significance in the PALACE 1-3 trials. 5 Pooled PALACE 1-3 ACR20, ACR50, and ACR70 responses in patients at week 52 were 55%, 26%, and 12%, respectively. 6 This is exploratory and has not been adjusted for multiple comparisons. Otezla has an established safety profile. In the PALACE 1-3 trials, the most common adverse reactions in at least 5% of patients receiving Otezla through week 16 were: diarrhea, nausea, and headache. 5 Treatment was discontinued due to any adverse reactions in 4.6% of Otezla treated patients and 1.2% of placebo treated patients. 5 Postmarketing reports of severe diarrhea, nausea, and vomiting have been associated with the use of Otezla. In some cases patients were hospitalized. Monitor patients who are more susceptible to complications of diarrhea or vomiting. 5 The most common adverse reactions that occurred at week 52 in at least 5% of patients were consistent with those observed through week 52. 6 Let’s dive into the cDAPSA post-hoc analysis data from the PALACE 1-3 trials. cDAPSA was retrospectively evaluated in 494 patients receiving Otezla 30 mg twice daily who had undergone randomization who had cDAPSA components available at baseline. 4 Outcome measures included the probability of achieving cDAPSA treatment targets, defined as achieving either remission or low disease activity, LDA, at week 52. 4 Let’s first look at the cDAPSA longitudinal assessment of disease activity through week 52. This assessment retrospectively analyzed pooled patients within each cDAPSA disease activity category at week 52 to determine the mean cDAPSA scores at baseline. 4 We can see that those who achieved treatment targets at week 52 had lower cDAPSA scores at baseline versus patients who did not. 4 Now, let’s discuss the percentage of patients who achieved cDAPSA treatment targets at week 52 according to their disease activity at baseline. Almost half of the patients with moderate disease activity at baseline achieved treatment targets of remission or LDA by week 52… 4 and approximately 37% achieved treatment targets at week 16. 4 We are now approaching the end of this video. Before I summarize the key take-home messages, let’s listen to the important safety information for Otezla.
VO: Warnings and Precautions. Hypersensitivity: Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapy. Diarrhea, Nausea, and Vomiting: Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized. Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications from severe diarrhea, nausea, or vomiting. Monitor patients who are more susceptible to complications of diarrhea or vomiting; advise patients to contact their healthcare provider. Consider Otezla dose reduction or suspension if patients develop severe diarrhea, nausea, or vomiting. Depression: Carefully weigh the risks and benefits of treatment with Otezla for patients with a history of depression and/or suicidal thoughts or behavior, or in patients who develop such symptoms while on Otezla. Patients, caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and they should contact their healthcare provider if such changes occur. Treatment with Otezla is associated with an increase in depression. During clinical trials, 1.0% reported depression or depressed mood compared to 0.8% treated with placebo. Suicidal ideation and behavior was observed in 0.2% of patients on Otezla, compared to none in placebo-treated patients. Depression was reported as serious in 0.2% of patients exposed to Otezla, compared to none in placebo-treated patients. Two patients who received placebo committed suicide compared to none on Otezla. Weight Decrease: Monitor body weight regularly; evaluate unexplained or clinically significant weight loss, and consider discontinuation of Otezla. Body weight loss of 5 to 10% was reported in 10% of patients taking Otezla and in 3.3% of patients taking placebo. Drug Interactions: Apremilast exposure was decreased when Otezla was co-administered with rifampin, a strong CYP450 enzyme inducer; loss of Otezla efficacy may occur. Concomitant use of Otezla with CYP450 enzyme inducers (for example, rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended. Adverse Reactions. The most common adverse reactions (5% or more) are diarrhea, nausea, and headache. Use in Specific Populations. Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss. Please see the Full Prescribing Information for Otezla provided on OtezlaPro.com.
Dr. Sulich: As noted previously, this post hoc analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn. In conclusion, this short video highlighted Otezla data from the PALACE 1-3 trials and a cDAPSA post-hoc analysis that identified which patients were likely to achieve treatment targets based on disease activity at baseline. 4 Thank you for listening!
References: 1. Kavanaugh, et al. Ann Rheum Dis 2014;73:1020-1026. 2. Cutolo, et al. J Rheumatol. 2016;43:9. 3. Edwards, et al. Ann Rheum Dis. 2016;75:1065-1073. 4. Mease, et al. Arthritis Care Res (Hoboken). 2020;72:814-821. 5. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 6. Kavanaugh, et al. Arthritis Res Ther. 2019;21:118.
Post-hoc analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
†††Post-hoc analyses were performed using multiple imputation for discontinuations and missing values. ‡‡‡Includes patients randomized to receive Otezla 30 mg BID at baseline and who had available cDAPSA components at week 52 (n=375). §§§The probability of achieving cDAPSA categories at week 52 was evaluated on the basis of baseline and week 16, with cDAPSA disease activity categories defined as follows: REM score ≤4, LDA score >4 to ≤13, MDA score >13 to ≤27, and HDA score >27. **** Clinical remission does not mean drug-free remission or complete absence of disease. ††††cDAPSA was calculated as a composite score based on SJC, TJC, PAP, and PtGA with possible scores ranging from 0 to 154.
‡‡‡‡Post-hoc analyses were performed using multiple imputation for discontinuations and missing values. §§§§Includes patients randomized to receive Otezla
30 mg BID at baseline and who had available cDAPSA components at week 52 (n=138). *****The probability of achieving cDAPSA categories at week 52 was
evaluated on the basis of baseline and week 16, with cDAPSA disease activity categories defined as follows: REM score ≤4, LDA score >4 to ≤13, MDA
score >13 to ≤27, and HDA score >27.
††††† Clinical remission does not mean drug-free remission or complete absence of disease. ‡‡‡‡‡cDAPSA was calculated
as a composite score based on SJC, TJC, PAP, and PtGA with possible scores ranging from 0 to 154.
ACR, American College of Rheumatology; BID, twice daily; cDAPSA, clinical Disease Activity Index for Psoriatic Arthritis; DMARDs, disease-modifying antirheumatic drugs; CRP, C-reactive protein; FAS, full analysis set; HDA, high disease activity; LDA, low disease activity; MDA, moderate disease activity; REM, remission; SJC, swollen joint count; TJC, tender joint count.
Contraindications
Otezla is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity: Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Please click here for the full Prescribing Information.
Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for
phototherapy or systemic therapy.
Otezla is indicated for the treatment of adult patients with active psoriatic arthritis.
Otezla is indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Wells AF, Edwards CJ, Kivitz AJ, et al. Rheumatology (Oxford). 2018;57(7):1253-1263. 4. Mease PJ, Kavanaugh A, Ogdie A, et al. J Rheumatol. Published online April 15, 2022. doi: 10.3899/jrheum.210906 5. Mease PJ, Gladman DD, Ogdie A, et al. Arthritis Res Care (Hoboken). 2020;72(6):814-821. 6. Schoels MM, Aletaha D, Alasti F, et al. Ann Rheum Dis. 2016;75(5):811-818. 7. Ogdie A, Coates LC, Gladman DD. Rheumatology (Oxford). 2020;59(suppl 1):i37-i46. 8. Singh JA, Guyatt G, Ogdie A, et al. Arthritis Rheum. 2019;71(1):5-32. 9. Mease PJ, Kavanaugh A, Ogdie A, et al. Probability of achieving low disease activity or remission in subjects with active PsA treated with apremilast. Presented at: the 2020 ACR Annual Meeting; November 5-9, 2020.