First and only oral therapy approved for mild, moderate, and severe plaque psoriasis, and active PsA SEE THE DATA

Plaque Psoriasis
3 INDICATIONS Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for phototherapy or systemic therapy. Read more
*Estimates of patients treated reflect global data since launch (Apr 2014-Mar 2023; US=58% of data). Calculations based on observed drug utilization parameters and number of units distributed. Utilization patterns change over time to best represent current markets.
FDA, U.S. Food and Drug Administration; PsA, psoriatic arthritis; TB, tuberculosis.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Data on file, Amgen Inc. 3. Otezla® (apremilast) FDA approval letter. March 21, 2014.
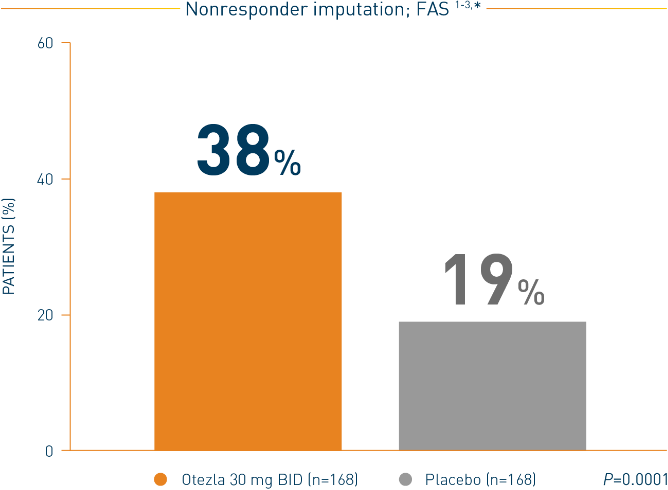
Otezla also significantly improved ACR20 response vs placebo at week 16 in PALACE 2 and 3; nonresponder imputation; intent-to-treat analysis 1,2
*FAS consists of all patients who were randomized as specified in the protocol.
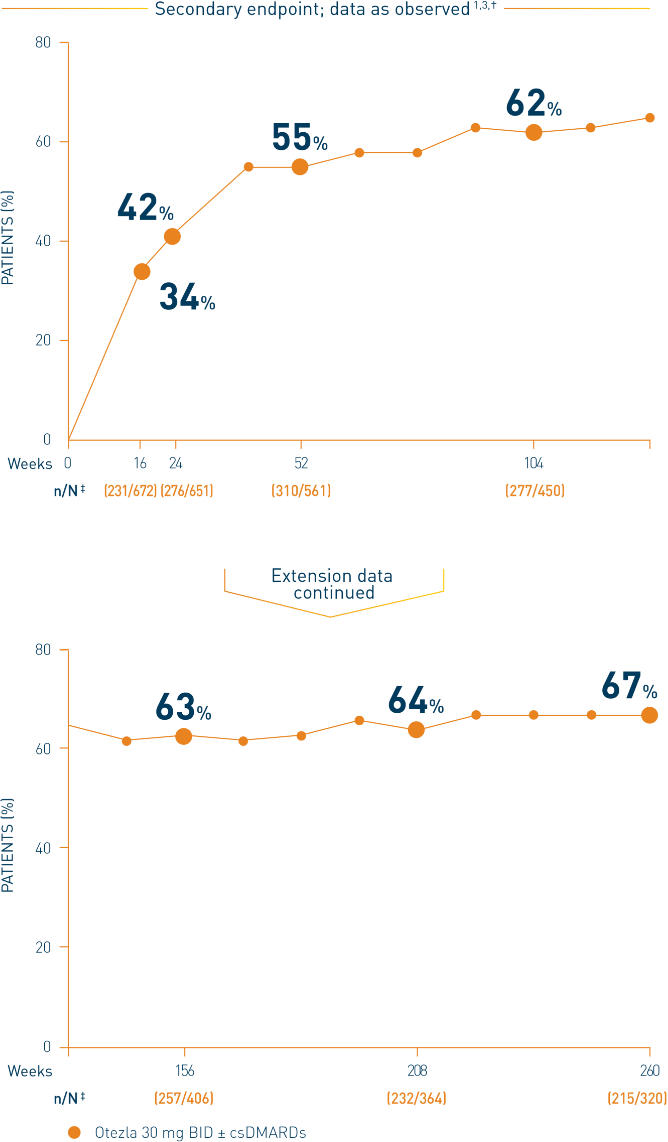
†Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. ‡n/N, number of responders/number of patients who had sufficient data for a definitive determination of response status at the timepoint, which includes patients who discontinued early between the preceding timepoint and the specific timepoint.
§The OLE period was from weeks 52 to 260.
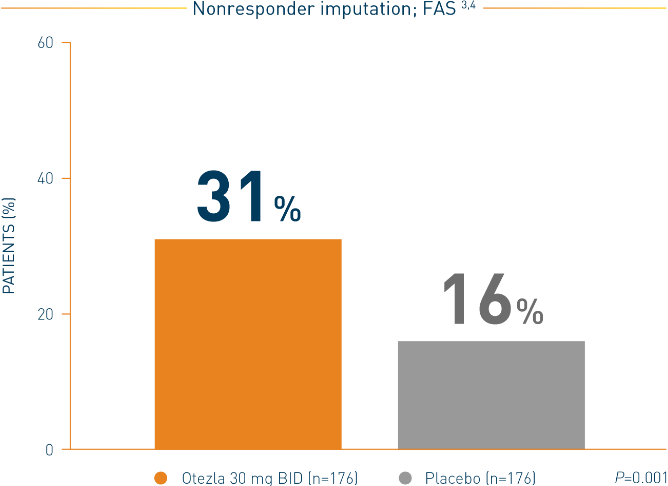
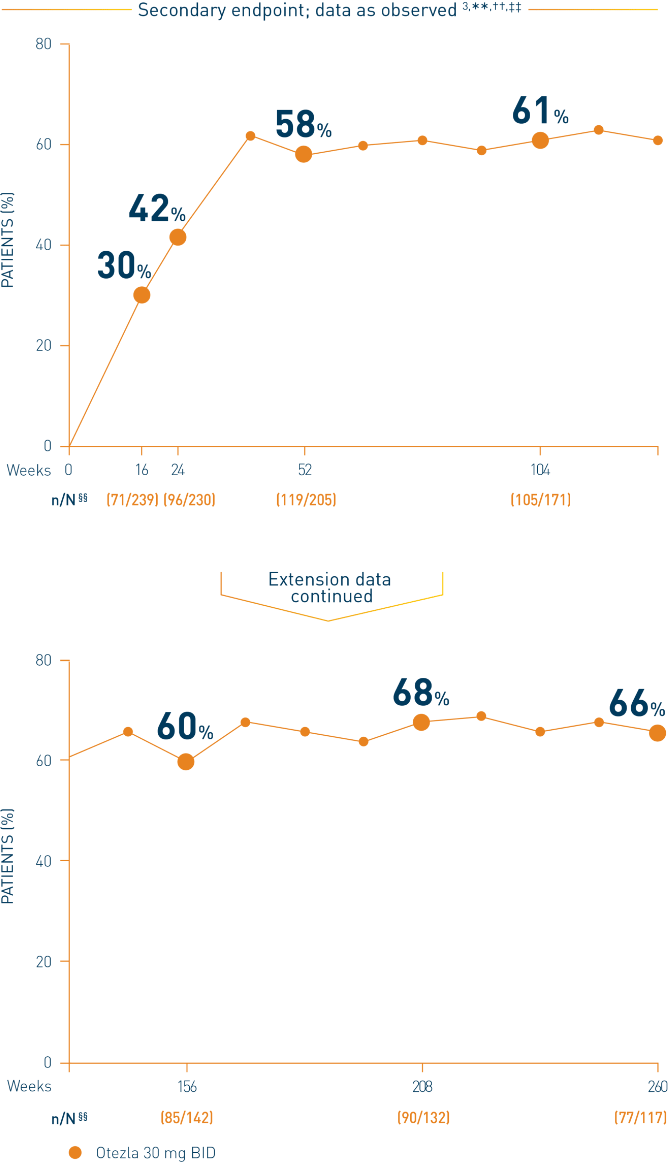
**Includes all patients exposed to Otezla, including during the placebo-controlled period, regardless of when patients started taking Otezla (baseline, week 16, or week 24) through week 260. ††Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 260 weeks. ‡‡Patients discontinued treatment during the study due to adverse reactions, lack of efficacy, and other (withdrawal by patient, loss of follow-up, protocol violation, noncompliance, and other). §§n/N, number of responders/number of subjects who had sufficient data for a definitive determination of response status at the timepoint, which includes subjects who discontinued early between the preceding timepoint and the specific timepoint.
***The OLE period was from weeks 52 to 260.
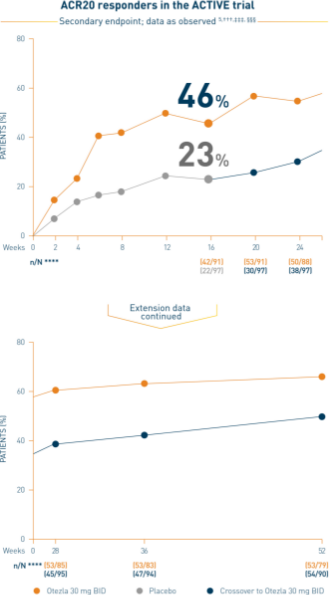
†††Data are presented “as observed” with no imputation for missing values in order to describe outcomes among those patients who continued to receive treatment over 52 weeks. ‡‡‡The n at each timepoint represents patients with a baseline value and a post-baseline value at the timepoint and includes subjects who discontinued early between the preceding timepoint and the specific timepoint. §§§Includes all patients exposed to Otezla from baseline who completed treatment through week 52. ****At week 16, patients receiving placebo were eligible to be switched to Otezla; at week 24, all remaining patients receiving placebo were switched to Otezla.
Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn. 3
ACR, American College of Rheumatology; BID, twice daily; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DMARD, disease-modifying antirheumatic drug; FAS, full analysis set; PsA, psoriatic arthritis.
Contraindications
Otezla is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulationWarnings and Precautions
Hypersensitivity: Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported during postmarketing surveillance. If signs or symptoms of serious hypersensitivity reactions occur, discontinue Otezla and institute appropriate therapyContraindications
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
Please click here for the full Prescribing Information.
Otezla® (apremilast) is indicated for the treatment of adult patients with plaque psoriasis who are candidates for
phototherapy or systemic therapy.
Otezla is indicated for the treatment of adult patients with active psoriatic arthritis.
Otezla is indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease.
References: 1. Otezla [package insert]. Thousand Oaks, CA: Amgen Inc. 2. Kavanaugh A, Gladman DD, Edwards CJ, et al. Arthritis Res Ther. 2019;21(1):118. 3. Data on file, Amgen Inc. 4. Wells AF, Edwards CJ, Kivitz AJ, et al. Rheumatology (Oxford). 2018;57(7):1253-1263. 5. Nash P, Ohson K, Walsh J, et al. Ann Rheum Dis. 2018;77(5):690‑698.